Your IND program.
Fully managed, end-to-end.
From CMC and toxicology to regulatory strategy and vendor coordination — we guide your asset to IND submission without the operational drag.
Why clients choose this program
A cleaner path to IND, with fewer risks and better leverage.
Single contract — no multi-vendor mess
Proven-capable custom-fit vendors
Avoid tech transfer disruptions
Faster execution and better cost control
What’s included in our IND support
Everything your asset needs to reach IND — coordinated under one roof.

Drug substance (API) development
End-to-end API sourcing, development, and scale-up with vetted CDMOs experienced in your molecule type.
Regulatory strategy and GAP analysis
Early alignment with FDA/EMA expectations to identify data gaps and define your path to a successful IND.
Drug product manufacturing
From formulation to fill-finish — we coordinate drug product production using compatible, audit-ready partners.

Weekly program management and reporting
We oversee timelines, deliverables, and vendor coordination — so you’re always in the loop without chasing updates.

IND-enabling toxicology and safety studies
We manage GLP-compliant tox studies across multiple species and routes, aligned with your clinical indication.
Audit Support, QA/QC Oversight
Full transparency and compliance management, including vendor audits and data validation at every step.
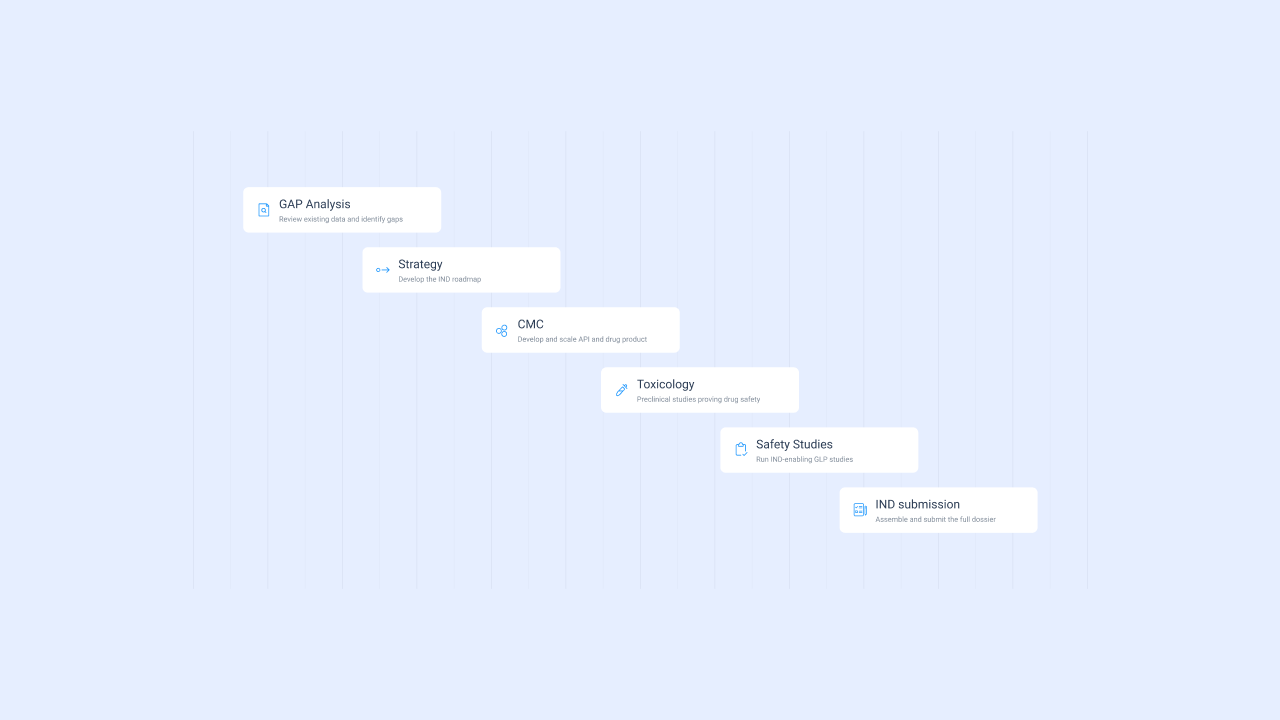
How it works – from start to IND submission
We don’t repurpose generic teams. For every program, we assemble a custom group of specialists — from regulatory leads and program managers to QA experts and clinicians — tailored to your asset’s complexity and development phase.
GAP analysis and program design
We assess your current data, identify gaps, and create a tailored plan to IND.
Custom team and vendor matching
We assemble your expert team and match you with vetted CDMOs and CROs based on your asset and modality.
Execution and coordination
Toxicology, CMC, and formulation work are executed in parallel — with weekly coordination and status tracking.
Submission and regulatory oversight
Final documentation is reviewed and submitted with full QA and regulatory support through Davos.
Additional services
Supporting services that give you flexibility, oversight, and control.
Global logistics & documentation
SEC-compliant tracking & reporting
Due diligence for fundraising or M&A
Insurance for materials and shipping
Transforming drug development with proven success
Our Integrated IND Platform streamlines workflows, ensuring faster and more reliable outcomes. Experience unparalleled efficiency and effectiveness in drug development.
INDs filed to date.
Success Rate (0 clinical holds).
Let's discuss your program
Contact us today to learn how our Integrated IND Platform can streamline your projects.
Explore our Case Studies
Real-world applications of our services
FAQ's
Find answers to common questions about our pharmaceutical services and solutions.
DavosPharma is a leading provider of pharmaceutical services. We specialize in drug development outsourcing. Our goal is to streamline your drug development process.
Our Integrated IND Platform brings together global CDMOs, CROs, and expert consultants to manage every step — from CMC, toxicology, to regulatory filing — so you can open an IND faster with less overhead.
We offer a range of services including Discovery, Biologics, Small Molecule, Drug Product, and Toxicology. Each service is designed to meet the unique needs of our clients. Our expertise ensures high-quality outcomes.
Vendor matchmaking connects you with the right partner for your project. We align your budget, technical needs, and goals with our global network of vetted vendors — optimizing efficiency and maximizing the likelihood of success.
You can reach us through our Contact page. Fill out the lead form, and we will get back to you promptly. We look forward to discussing your needs.
Still have questions?
We're here to help you with any inquiries.


